Product Usage: This product is intended as a research chemical only. This designation permits the use of research chemicals strictly for in vitro testing and laboratory research, but not for human or animal.. This product should only be handled by licensed, qualified professionals. All information available on this website is for educational purposes only. This product is not a drug, food, or cosmetic and may not be misbranded, misused, or mislabeled as such. Acknowledged to the Terms and Conditions, the customer hereby agrees to take full responsibility on how to use the product.







Description
xCerebrolysin is a nootropic drug, which means that it has the capacity to enhance a number of cognitive functions such as memory, concentration, and thinking skills. It is used in the treatment of memory disorders, concentration disorders, and degenerative dementia, including Alzheimer’s disease. Cerebrolysin is also used in the treatment of acute neurological disorders, such as cerebral stroke and craniocerebral trauma. The brain-boosting effects of cerebrolysin may be attributed to the neuropeptides it contains. These neuropeptides are active brain peptides (chains of amino acids) that are used by nerve cells (neurons) to enhance their communication with each other.
Cerebrolysin is a natural compound derived from the brains of pigs using a safe and standardized enzymatic process. In order to achieve its therapeutic effect, cerebrolysin needs to be administered in the form of injections.
How Cerebrolysin Works
Cerebrolysin works by increasing the levels of neurotrophic factors (NFT) and brain-derived neurotrophic factors (BDNF). This in turn stimulates the formation and repair of neurons (nerve cells) in the brain.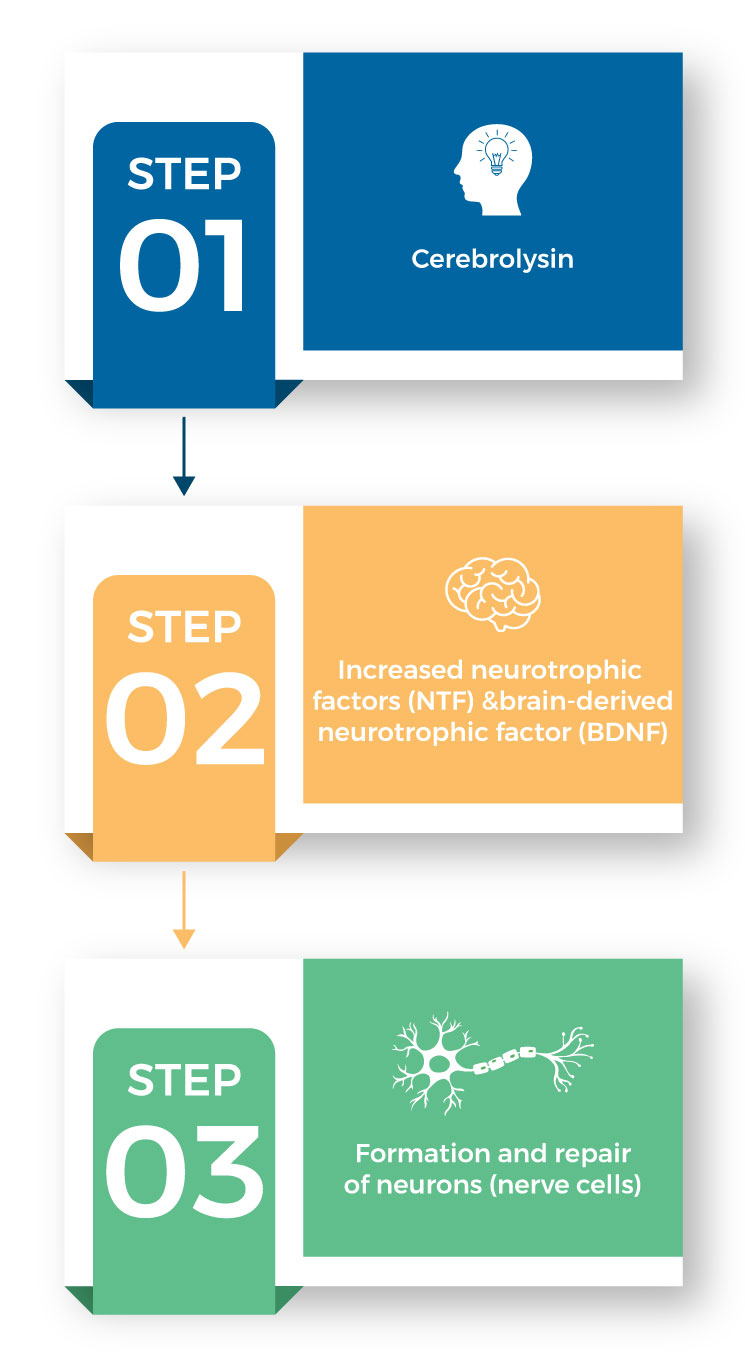
Chemical Structure of Cerebrolysin
Research on Cerebrolysin
A. Boosts Cognitive Function
There is increasing evidence that cerebrolysin may help improve cognitive function and counter the effects of certain medical conditions that lead to cognitive impairment:
- In patients with schizophrenia dominated by negative symptoms, administration of cerebrolysin in addition to antipsychotic medication appears to improve cognitive function and memory. [1]
- In healthy older adults with memory loss, the administration of a derivative of cerebrolysin, N-PEP-12, improved memory. [2]
- In healthy elderly adults, a single dose of cerebrolysin improved memory performance.[3]
- In patients with Alzheimer’s disease and dementia, high doses of cerebrolysin reduced psychological symptoms and slowed disease progression. [4]
- In animals with Alzheimer’s disease, cerebrolysin administration reduced the build-up of brain beta-amyloid plaques, which are sticky proteins known to cause the disease. [5-7]
- In patients with stroke and traumatic brain injury (TBI), cerebrolysin administration appears to improve cognitive recovery without any adverse side effects. [8-14]
- In infants with communication defects due to severe brain damage during pregnancy, cerebrolysin treatment for 3 months improved communication and social interaction.[15]
- In mouse and rat models of Parkinson’s disease, cerebrolysin promoted survival of brain cells, improved motor symptoms, and slowed disease progression. [16-18]
- The combination of cerebrolysin with recombinant tissue-Plasminogen Activator produced a more favorable response in neurological outcome measures as compared to the placebo group. [19]
- In patients with stroke who were treated with cerebrolysin (30 mL over seven days followed by 10 mL until day 30) once daily over a period of four weeks, a significant improvement in neurological and global function outcomes was observed compared to the group who received placebo treatment. [20]
- In dementia models and stroke animal models, cerebrolysin decreased the accumulation of abnormal protein structures in the brain, improved the transmission of signals by the neurons (nerve cells), restored neuron structures, induced restorative processes, decreased infarct volume (dead tissue) and formation of edema (swelling), resulting in improved cognitive and behavioral performance. [21-23]
- In patients with ischemic stroke (insufficient blood flow to the brain), the administration of cerebrolysin produced significant improvements in motor and cognitive recovery. [24-28]
- In elderly patients with vascular dementia of mild to moderate severity, cerebrolysin administration produced beneficial effects on general cognitive function as measured by mini-mental state examination (MMSE). [29]
B. Improves Mood
The brain-boosting effect of cerebrolysin also has a beneficial effect on mood, especially in depressive symptoms. Studies show that administration of cerebrolysin produces an antidepressant effect:
- In elderly patients with depression, intravenous infusions of cerebrolysin reduced symptoms of depression, anxiety, and apathy (lack of interest, enthusiasm, or concern). [30]
- In patients with Alzheimer’s disease, cerebrolysin treatment improved scores on the Cornell Depression Scale. [31]
- In patients with treatment-resistant depression, cerebrolysin therapy improved depressive symptoms without any adverse side effects. [32]
- In rats, cerebrolysin administration produced an anti-anxiety effect. [33]
C. Improves Symptoms of Autism
Autism, or autism spectrum disorder (ASD), refers to a wide range of medical conditions that affect social interaction, behavior, speech, and nonverbal communication. Since cerebrolysin has the capacity to enhance a number of cognitive functions, this nootropic drug has also been studied for its beneficial effect on autism:
- In patients with childhood autism and Asperger’s syndrome (one of the autism spectrum disorders), cerebrolysin therapy improved various areas of cognitive function (expressive and receptive speech, fine motoring, and playing). [34]
- In children with autism, cerebrolysin therapy improved symptoms and scores on the Childhood Autism Rating Scale (CARS). [35]
- In a rat model of autism, cerebrolysin therapy improved behavior and brain cell communication. [36]
D. Improves Symptoms of Attention Deficit Hyperactivity Disorder (ADHD)
ADHD is a mental disorder characterized by hyperactivity, impulsivity, and short attention span. This mental disorder affects children and teens and can transition into adulthood. Studies show that administration of cerebrolysin may help reduce symptoms of ADHD:
- In children with ADHD, cerebrolysin administration at a dose of 1 ml per 10 kg of weight intramuscularly for 1 month improved the core symptoms of ADHD. [37]
- In patients with ADHD of unknown cause, cerebrolysin treatment reduced impulsivity and hyperactivity. [38]
E. Improves Symptoms of Cerebral Palsy (CP)
- In patients with CP, intramuscular administration of cerebrolysin as a single daily dose of 0.1 cc/kg for 10 days and then continued weekly for 4 months appears to improve gross motor function. [39]
- In patients with infantile cerebral paralysis, the administration of cerebrolysin improved symptoms without any adverse side effects. [40]
- In pediatric patients living with CP, the combination of standard rehabilitation therapy with cerebrolysin improved gross motor skills. [41]
- In children with traumatic brain injury and cerebral palsy, cerebrolysin therapy induced the repair of nerve cells of the brain. [42]
F. Repairs Nerve Damage
With nerve damage, there can be a broad range of symptoms depending on the location and types of nerves affected. In addition, chronic nerve damage may impair the sensation or function of the affected body part. Interestingly, numerous studies support the therapeutic benefits of cerebrolysin in different medical conditions associated with nerve damage:
- In patients with diabetic neuropathy (nerve damage caused by diabetes), daily cerebrolysin infusion over a period of 10 days alleviated pain and tingling sensations. [43]
- In a mouse model of diabetic neuropathy, the administration of cerebrolysin improved dysfunction of the sciatic nerve (the largest single nerve located on each side of the lower spine going all the way down to the foot). [44]
- In patients with different nerve injuries, cerebrolysin treatment was associated with rapid neurological recovery after various peripheral nerve lesions than conventional therapies. [45]
- In aged rats, cerebrolysin treatment ameliorated performance deficits and nerve damage. [46]
- In mice, low-dose cerebrolysin administration promoted regeneration of injured spinal motor neurons. [47]
- In animals with nerve injury related to microsurgical suturing, cerebrolysin administration promoted regeneration of the injured peripheral nerve. [48]
- In patients with various forms of nerve injury, cerebrolysin was more associated with rapid recovery of neurological functions than other therapies such as steroids and supportive therapies such as vitamins and antioxidants. [49]
G. Treats Hyperthermia-Induced Neurotoxicity
Adverse environmental circumstances such as heat stress related to hot climates can lead to disturbed mental function. This condition is known as hyperthermia-induced neurotoxicity. Researchers suggest that one of the suitable therapeutic strategies to treat heat-induced mental anomalies related to this condition is cerebrolysin administration. Studies show that cerebrolysin exerts its therapeutic effect through the following important mechanisms:
- In rats exposed to whole body hyperthermia (increased temperature), cerebrolysin administration induced a marked reduction in brain toxicity, thus preventing mental dysfunction related to heat stress. [50]
- In rats suffering from heat stroke, cerebrolysin exerted superior neuroprotective effects as compared to other neuroprotective agents. [51]
H. Treats Morphine Withdrawal Symptoms
Prolonged use of morphine changes the way nerve receptors in the brain work. As a result, sudden withdrawal from this drug can lead to debilitating symptoms such as sleep problems, restlessness, anxiety, digestive problems, high blood pressure, rapid heartbeat, and vision problems. Studies suggest that cerebrolysin can be considered a therapeutic option for morphine withdrawal symptoms:
- A study reported that morphine withdrawal can activate heat shock protein which triggers unpleasant symptoms, suggesting that cerebrolysin can produce beneficial effects through its ability to suppress the activation of these proteins. [52]
- Rat studies showed that cerebrolysin counteracted the activation of heat shock protein, thus alleviating the negative effects of morphine withdrawal. [53-54]
I. Boosts Immune Function
There is mounting evidence that cerebrolysin may help heighten the immune response and prevent a wide array of diseases. Studies show that cerebrolysin positively affects the production of different cells of the immune system:
- In mice, cerebrolysin treatment increased the production of Thy-1 cells. [55]
- In patients with mental developmental delay, cerebrolysin therapy at a dose of 0.1 mg/1kg of body mass for 42 days improved immune status. [56]
- In patients with minimal cerebral dysfunction, intrasmuscular cerebrolysin administration at a dose of 1 ml per 10 kg of body weight for 1 month increased the blood levels of CD19(+) and CD16(+) cells with a simultaneous normalization of blood IgG, IgA, and natural killer cell levels. [57]
- A laboratory study showed that cerebrolysin exerts its immune-boosting properties by increasing the production of T- and B-lymphocytes, and promoting the survival of immunocompetent cells. [58]
- Studies found that cerebrolysin can help decrease the levels of free radicals, which are unstable molecules that damage cells and impair the function of immune system cells. [59-62]
J. Improves Eye Health
IMG
Cerebrolysin has the capacity to stimulate the regeneration of various nerves and cells in the body. Studies show that this regenerative ability may help maintain visual health:
- In a rat model of optic nerve crush, the injection of cerebrolysin promoted the survival of retinal cells. [63]
- In a patient with vision loss in both eyes, intense photophobia (light sensitivity), and eye pain, cerebrolysin administration appears to reduce eye pressure and improve visual acuity. [64]
Associated Side Effects and Adverse Events of Cerebrolysin
Cerebrolysin side effects are very uncommon, but adverse events have been reported. There is also a potential for serious adverse events with Cerebrolysin use, including an increase in non-fatal serious adverse events. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on cerebrolysin. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of cerebrolysin. Despite this, it was listed as a side effect associated with cerebrolysin even though these associated side effects are very uncommon.
Side effects associated with cerebrolysin may include the following:
- Agitation
- Changes in blood pressure
- Confusion
- Constipation
- Diarrhea
- Fatigue
- Feeling hot
- Nausea
- Seizure
- Vertigo
- Vomiting
Cortexin ® vs Cerebrolysin ®
Cortexin® and Cerebrolysin® are both peptide-based drugs used primarily in neurology for their neuroprotective and neurotrophic effects, but they have distinct compositions and slightly different applications. Cortexin® is derived from the cerebral cortex of pigs and contains a complex of polypeptide fractions along with amino acids that influence the central nervous system. It is administered to improve brain function, protect against damage, and enhance recovery from various neurological conditions. Unlike Cerebrolysin, which is derived from pig brain proteins and contains a mixture of low-molecular-weight peptides and free amino acids, Cortexin’s active components are smaller and potentially more focused in their action.
In terms of clinical use, both Cortexin® and Cerebrolysin are utilized to treat similar conditions, including traumatic brain injuries, stroke recovery, and cognitive disorders such as Alzheimer’s disease. However, the specific indications and the perceived efficacy can vary. Cortexin is often noted for its neuroprotective properties and its ability to stabilize cell membranes and reduce oxidative stress. Cerebrolysin, on the other hand, is more explicitly recognized for its role in enhancing cognitive functions and supporting neuronal growth and repair, making it particularly useful in the treatment of dementia and similar degenerative conditions.
The choice between Cortexin® and Cerebrolysin often comes down to clinical objectives, patient response to treatment, and the preferences of the healthcare provider. Both medications are administered via injection, requiring similar protocols for use. Side effects for both drugs are generally mild but can include discomfort at the injection site, dizziness, and headaches. While both treatments are supported by a substantial amount of research, the body of evidence is more robust for Cerebrolysin, particularly in its effects on a broad range of neurodegenerative and cognitive disorders, as documented in the database of systematic reviews. Ultimately, the decision to use Cortexin or Cerebrolysin should be tailored to the individual patient’s condition and needs, often guided by the experience and observation of their healthcare team. Consulting the database of systematic reviews can provide valuable insights into the comparative efficacy and safety of these treatments. Additionally, continuous reference to the database of systematic reviews helps ensure that treatment decisions are based on the most comprehensive and up-to-date evidence available.
Cerebrolysin in Stroke and Vascular Dementia
Cerebrolysin has garnered attention in the medical community for its potential therapeutic effects in treating stroke and vascular dementia. This peptide-based treatment is believed to confer neuroprotective and neurotrophic benefits, making it a viable option for promoting neural repair and functional recovery post-stroke. The neurotrophic factors present in Cerebrolysin are thought to enhance neurogenesis, reduce inflammation, and improve synaptic connectivity, which can be critical in the acute phase following a stroke. Clinical trials and studies have shown that when administered soon after a stroke, Cerebrolysin can help improve neurological function and reduce the extent of brain damage, with a low incidence of serious adverse events.
In the context of vascular dementia, Cerebrolysin’s ability to improve cognitive functions and protect neural structures offers a promising approach to managing this condition. Vascular dementia, which results from impaired blood flow to the brain leading to cognitive decline, can benefit from Cerebrolysin’s mechanisms that enhance cerebral blood flow and neuronal resilience. Patients treated with Cerebrolysin have reported improvements in memory, attention, and executive function. Continuous research and clinical trials suggest that Cerebrolysin not only helps in stabilizing the symptoms of vascular dementia but may also slow its progression, offering a better quality of life for patients, with minimal serious adverse events.
Despite the promising outcomes, the use of Cerebrolysin in stroke recovery and vascular dementia must be carefully considered by healthcare professionals. The treatment involves a series of injections or infusions, which require monitoring and management by medical personnel. Side effects, though generally mild, can include headache, nausea, and dizziness. The risk of serious adverse events, while low, requires careful monitoring. Reviews in the Cochrane Database of Systematic Reviews highlight the importance of such vigilance in clinical practice. The cost of treatment and the variability in patient responses also pose challenges. Therefore, while Cerebrolysin offers a potentially effective treatment modality for stroke and vascular dementia, it should be part of a comprehensive therapeutic plan that includes other medical interventions and lifestyle adjustments tailored to individual patient needs. Ongoing vigilance for any serious adverse events is critical, as emphasized by findings from the Cochrane Database of Systematic Reviews. Additionally, systematic reviews and meta-analyses, such as those found in the Cochrane Database of Systematic Reviews, provide valuable insights into the efficacy and safety of Cerebrolysin, guiding healthcare professionals in making informed decisions about its use in diverse patient populations.
Reference
-
Xiao S, Xue H, Li G. Therapeutic effects of cerebrolysin added to risperidone in patients with schizophrenia dominated by negative symptoms. The Australian and New Zealand journal of psychiatry. 2012; 46(2):153-60. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22311531
-
Crook TH, Ferris SH, Alvarez XA, Laredo M, Moessler H. Effects of N-PEP-12 on memory among older adults. International clinical psychopharmacology. 2005; 20(2):97-100. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15729085
-
Alvarez XA, Lombardi VR, Corzo L. Oral Cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. Journal of neural transmission. Supplementum. 2000; 59:315-28. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10961443
-
Allegri RF, Guekht A. Cerebrolysin improves symptoms and delays progression in patients with Alzheimer’s disease and vascular dementia. Drugs of today (Barcelona, Spain: 1998). 2012; 48 Suppl A:25-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22514793
-
Rockenstein E, Torrance M, Mante M. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease. Journal of neuroscience research. 2006; 83(7):1252-61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16511867
-
Rockenstein E, Mallory M, Mante M. Effects of Cerebrolysin on amyloid-beta deposition in a transgenic model of Alzheimer’s disease. Journal of neural transmission. Supplementum. 2002. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12456076.
-
Rockenstein E, Adame A, Mante M, Moessler H, Windisch M, Masliah E. The neuroprotective effects of Cerebrolysin in a transgenic model of Alzheimer’s disease are associated with improved behavioral performance. Journal of neural transmission (Vienna, Austria : 1996). 2003; 110(11):1313-27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14628195
-
Ladurner G, Kalvach P, Moessler H, .Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial. Journal of neural transmission (Vienna, Austria : 1996). 2005; 112(3):415-28. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15583955
-
Muresanu DF, Heiss W-D, Hoemberg V, et al. Cerebrolysin and Recovery After Stroke (CARS): A Randomized, Placebo-Controlled, Double-Blind, Multicenter Trial. Stroke; a Journal of Cerebral Circulation. 2016;47(1):151-159. doi:10.1161/STROKEAHA.115.009416. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4689177/.
-
Guekht A, Vester J, Heiss WD. Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta-analysis of the CARS trials. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2017; 38(10):1761-1769. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28707130.
-
Chen CC, Wei ST, Tsaia SC, Chen XX, Cho DY. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. British journal of neurosurgery. 2013; 27(6):803-7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23656173.
-
Bornstein N, Poon WS. Accelerated recovery from acute brain injuries: clinical efficacy of neurotrophic treatment in stroke and traumatic brain injuries. Drugs of today (Barcelona, Spain : 1998). 2012; 48 Suppl A:43-61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22514794
-
Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Actaneurochirurgica. Supplement. 2005; 95:59-60. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16463821.
-
Zhang D, Dong Y, Li Y, Chen J, Wang J, Hou L. Efficacy and Safety of Cerebrolysin for Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials. BioMed Research International. 2017;2017:4191670. doi:10.1155/2017/4191670. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5474547/.
-
Hassanein SM, Deifalla SM, El-Houssinie M, Mokbel SA. Safety and Efficacy of Cerebrolysin in Infants with Communication Defects due to Severe Perinatal Brain Insult: A Randomized Controlled Clinical Trial. Journal of clinical neurology (Seoul, Korea). 2016; 12(1):79-84. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26365023.
-
Ozkizilcik A, Sharma A, Muresanu DF. Timed Release of Cerebrolysin Using Drug-Loaded TitanateNanospheres Reduces Brain Pathology and Improves Behavioral Functions in Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28875428.
-
Ozkizilcik A, Sharma A, Muresanu DF. Timed Release of Cerebrolysin Using Drug-Loaded TitanateNanospheres Reduces Brain Pathology and Improves Behavioral Functions in Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28875428.
-
Requejo C, Ruiz-Ortega JA, Cepeda H. Nanodelivery of Cerebrolysin and Rearing in Enriched Environment Induce Neuroprotective Effects in a Preclinical Rat Model of Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28840482.
-
Noor NA, Mohammed HS, Mourad IM, Khadrawy YA, AboulEzz HS. A promising therapeutic potential of cerebrolysin in 6-OHDA rat model of Parkinson’s disease. Life sciences. 2016; 155:174-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27210889
-
KalynIaB, Safarova TP, Sheshenin VC, Gavrilova SI. [Comparative efficacy and safety of antidepressant mono- and multimodal therapy in elderly patients with depression (a clinical experience in a psychogeriatric hospital)]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2014; 114(6 Pt 2):20-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25042499.
-
Gharagozli, K., Harandi, A. A., Houshmand, S., Akbari, N., Muresanu, D. F., Vester, J., Winter, S., & Moessler, H. (2017). Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo-controlled, double-blinded, multicenter clinical trial. Journal of medicine and life, 10(3), 153–160.
-
Masliah, E., & Díez-Tejedor, E. (2012). The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs of today (Barcelona, Spain: 1998), 48 Suppl A, 3–24. https://doi.org/10.1358/dot.2012.48(Suppl.A).1739716
-
Fiani, B., Covarrubias, C., Wong, A., Doan, T., Reardon, T., Nikolaidis, D., & Sarno, E. (2021). Cerebrolysin for stroke, neurodegeneration, and traumatic brain injury: review of the literature and outcomes. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 42(4), 1345–1353. https://doi.org/10.1007/s10072-021-05089-2
-
Zhang, C., Chopp, M., Cui, Y., Wang, L., Zhang, R., Zhang, L., Lu, M., Szalad, A., Doppler, E., Hitzl, M., & Zhang, Z. G. (2010). Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. Journal of neuroscience research, 88(15), 3275-3281. https://doi.org/10.1002/jnr.22495.
-
Bornstein, N. M., Guekht, A., Vester, J., Heiss, W. D., Gusev, E., Hömberg, V., Rahlfs, V. W., Bajenaru, O., Popescu, B. O., & Muresanu, D. (2018). Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 39(4), 629–640. https://doi.org/10.1007/s10072-017-3214-0.
-
Heiss, W. D., Brainin, M., Bornstein, N. M., Tuomilehto, J., Hong, Z., & Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators (2012). Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke, 43(3), 630–636. https://doi.org/10.1161/STROKEAHA.111.628537.
-
Chang, W. H., Lee, J., Shin, Y. I., Ko, M. H., Kim, D. Y., Sohn, M. K., Kim, J., & Kim, Y. H. (2021). Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits. Journal of personalized medicine, 11(6), 545. https://doi.org/10.3390/jpm11060545
-
Tran, L., Alvarez, X. A., Le, H. A., Nguyen, D. A., Le, T., Nguyen, N., Nguyen, T., Nguyen, T., Vo, T., Tran, T., Duong, C., Nguyen, H., Nguyen, S., Nguyen, H., Le, T., Nguyen, M., & Nguyen, T. (2022). Clinical Efficacy of Cerebrolysin and Cerebrolysin plus Nootropics in the Treatment of Patients with Acute Ischemic Stroke in Vietnam. CNS & neurological disorders drug targets, 21(7), 621–630. https://doi.org/10.2174/1871527320666210820091655
-
Stan, A., Birle, C., Blesneag, A., & Iancu, M. (2017). Cerebrolysin and early neurorehabilitation in patients with acute ischemic stroke: a prospective, randomized, placebo-controlled clinical study. Journal of medicine and life, 10(4), 216–222.
-
Chen, N., Yang, M., Guo, J., Zhou, M., Zhu, C., & He, L. (2013). Cerebrolysin for vascular dementia. The Cochrane database of systematic reviews, (1), CD008900. https://doi.org/10.1002/14651858.CD008900.pub2.
-
Lang, W., Stadler, C. H., Poljakovic, Z., Fleet, D., & Lyse Study Group (2013). A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy of combined treatment with alteplase (rt-PA) and Cerebrolysin in acute ischaemic hemispheric stroke. International journal of stroke: official journal of the International Stroke Society, 8(2), 95–104. https://doi.org/10.1111/j.1747-4949.2012.00901.x.
-
Panisset M, Gauthier S, Moessler H, Windisch M, .Cerebrolysin in Alzheimer’s disease: a randomized, double-blind, placebo-controlled trial with a neurotrophic agent. Journal of neural transmission (Vienna, Austria: 1996). 2002; 109(7-8):1089-104. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12111446.
-
Retrieved from http://www.wfsbp.org/doi/wfsbp2011-abstractscd/en/abstracts/10025.html.
-
Shabanov PD, Lebedev AA, Pavlenko VP, Ganapol’skiĭ VP. [Comparative study of behavioral effects of cortexin and cerebrolysine upon intraventricular and intraperitoneal administration in rats]. Eksperimental’naia i klinicheskaiafarmakologiia. ; 70(3):13-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17650626
-
Krasnoperova MG, Bashina VM, Skvortsov IA, Simashkova NV. [The effect of cerebrolysin on cognitive functions in childhood autism and in Asperger syndrome]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2003; 103(6):15-8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12872620.
-
Chutko LS, Yakovenko EA, Surushkina SY, Kryukova EM, Palaieva SV. [The efficacy of cerebrolysin in the treatment of autism spectrum disorders]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2017; 117(9):71-75. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29053124.
-
Cuevas-Olguin R, Roychowdhury S, Banerjee A. Cerebrolysin prevents deficits in social behavior, repetitive conduct, and synaptic inhibition in a rat model of autism. Journal of neuroscience research. 2017; 95(12):2456-2468. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28609577.
-
Sotnikova NY, Gromova OA, Novicova EA. Dual effect of cerebrolysin in children with attention deficit syndrome with hyperactivity: neuroprotection and immunomodulation. Russian journal of immunology: RJI : official journal of Russian Society of Immunology. 2002; 7(4):357-64. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12687248
-
Chutko LS, Yakovenko EA, Surushkina SY, Anisimova TI, Kropotov YD. [Clinical and neurophysiological heterogeneity of attention deficit hyperactivity disorder]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova.; 116(10):117-121. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27845323.
-
Nasiri J, Safavifar F. Effect of cerebrolysin on gross motor function of children with cerebral palsy: a clinical trial. ActaneurologicaBelgica. 2017; 117(2):501-505. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28074392.
-
Gershman RN, Vasilenko MA. [Use of cerebrolysin and ATP in treating infantile cerebral paralysis]. Pediatriiaakusherstvo i ginekologiia.. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1228606.
-
Retrieved from https://cerebralpalsynewstoday.com/2017/06/07/cerebrolysin-can-help-improve-motor-skills-in-cerebral-palsy-patients/.
-
Retrieved from https://clinicaltrials.gov/ct2/show/NCT02116348
-
Biesenbach G, Grafinger P, Eichbauer-Sturm G, Zazgornik J. [Cerebrolysin in treatment of painful diabetic neuropathy]. Wiener medizinischeWochenschrift (1946). 1997; 147(3):63-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9173675.
-
Dong H, Jiang X, Niu C, Du L, Feng J, Jia F. Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy. Neural Regeneration Research. 2016;11(1):156-162. doi:10.4103/1673-5374.175063. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4774211/
-
Masliah E, Armasolo F, Veinbergs I, Mallory M, Samuel W. Cerebrolysin ameliorates performance deficits, and neuronal damage in apolipoprotein E-deficient mice. Pharmacology, biochemistry, and behavior. 1999; 62(2):239-45. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9972690.
-
Keilhoff G, Lucas B, Pinkernelle J, Steiner M, Fansa H. Effects of cerebrolysin on motor-neuron-like NSC-34 cells. Experimental cell research. 2014; 327(2):234-55. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24997385.
-
Shchudlo NA, Shchudlo MM, Borisova IV. [The effect of cerebrolysin on the regeneration of the peripheral nerve depending on the scheme of paraneural administration]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2013; 113(12):76-80. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24430040.
-
Available from https://journals.lww.com/nrronline/Abstract/2011/06180/Cerebrolysin_as_a_nerve_growth_factor_for.10.aspx.
-
Sharma HS, Sharma A, Mössler H, Muresanu DF. Neuroprotective effects of cerebrolysin, a combination of different active fragments of neurotrophic factors and peptides on the whole body hyperthermia-induced neurotoxicity: modulatory roles of co-morbidity factors and nanoparticle intoxication. International review of neurobiology. 2012; 102:249-76. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22748833.
-
Sharma A, Muresanu DF, Mössler H, Sharma HS. Superior neuroprotective effects of cerebrolysin in nanoparticle-induced exacerbation of hyperthermia-induced brain pathology. CNS & neurological disorders drug targets. 2012; 11(1):7-25. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22229316.
-
Martínez-Laorden E, Hurle MA, Milanés MV, Laorden ML, Almela P. Morphine withdrawal activates hypothalamic-pituitary-adrenal axis and heat shock protein 27 in the left ventricle: the role of extracellular signal-regulated kinase. The Journal of pharmacology and experimental therapeutics. 2012; 342(3):665-75. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22647273.
-
Sharma HS, Ali SF, Patnaik R, Zimmermann-Meinzingen S, Sharma A, Muresanu DF. Cerebrolysin Attenuates Heat Shock Protein (HSP 72 KD) Expression in the Rat Spinal Cord Following Morphine Dependence and Withdrawal: Possible New Therapy for Pain Management. Current neuropharmacology. 2011; 9(1):223-35. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21886595.
-
Belokrylov GA, Malchanova IV. [Levamin and cerebrolysin as immunostimulants]. Biulleten’ eksperimental’noibiologii i meditsiny. 1992; 113(2):165-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1611065.
-
Govorin NV, Zlova TP, Akhmetova VV, Tarasova OA. [The pathophysiological analysis of cerebrolysin therapy of children with mental developmental delay caused by ecological factors]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2008; 108(5):51-5. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18577958.
-
Sotnikova NY, Gromova OA, Novikova EA, Burtsev EM. Immunoactive Properties of Cerebrolysin. Russian journal of immunology: RJI : official journal of Russian Society of Immunology. 2000; 5(1):63-70. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12687163.
-
Garmanchuk LV, Perepelitsyna EM, SidorenkoMv, Makarenko AN, Kul’chikov AE. [Cytoprotective effect of neuropeptides on immunocompetent cells (in vitro study)]. Eksperimental’naia i klinicheskaiafarmakologiia.; 72(4):28-32. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19803367
-
González ME, Francis L, Castellano O. Antioxidant systemic effect of short-term Cerebrolysin administration. Journal of neural transmission. Supplementum. 1998; 53:333-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9700669.
-
Gromova OA, Avdeenko TV, Burtsev EM, Skal’nyĭ AV, Solov’ev OI. [Effects of cerebrolysin on the oxidant homeostasis, the content of microelements and electrolytes in children with minimal brain dysfunction]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 1998; 98(1):27-30. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9505400.
-
González ME, Francis L, Castellano O. Antioxidant systemic effect of short-term Cerebrolysin administration. Journal of neural transmission. Supplementum. 1998; 53:333-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9700669.
-
Retrieved from http://europepmc.org/abstract/med/9505400.
-
Huang TL, Huang SP, Chang CH, Lin KH, Sheu MM, Tsai RK. Protective effects of cerebrolysin in a rat model of optic nerve crush. The Kaohsiung journal of medical sciences. 2014; 30(7):331-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24924838.
-
Retrieved from http://www.roneurosurgery.eu/atdoc/16CostinDCombined.pdf.
Shipping & Return
xReturns Policy
At Qualitide, we are committed to ensuring your satisfaction with our products. Our Satisfaction Guarantee policy allows you to return or exchange any item within 14 days of receiving your order if you are not completely satisfied. Return/Exchange Eligibility To be eligible for a return or exchange: The item must be in its original condition as received, unworn or unused, with tags attached, and in its original packaging. You must provide the receipt or proof of purchase..
If you need to return an item, simply login to your account, view the order using the "Complete Orders" link under the My Account menu and click the Return Item(s) button. We'll notify you via e-mail of your refund once we've received and processed the returned item.
Shipping
At QUALITIDE, we aim to make your shopping experience simple and smooth. Here’s how we handle shipping: Processing Time We process all orders within 0 to 1 business day. Orders placed after business hours, on weekends, or on holidays will be processed the next business day. Delivery Time Once processed, delivery takes 2 to 3 business days, depending on your location and the shipping carrier. Total Delivery Time From placing your order to receiving it, the total time is 2 to 4 business days, including both processing and shipping.
Disclaimer
xAll articles and product information provided on this website are for informational and educational purposes only. The products offered on Qualitides are intended solely for in-vitro research purposes. In-vitro studies ("in glass" studies) are conducted outside of the body. These products are not medicines or drugs and have not been evaluated or approved by the FDA to prevent, treat, diagnose, or cure any medical condition, ailment, or disease. Any form of bodily introduction or administration of these products into humans or animals is strictly prohibited by law. By using this website, you acknowledge and agree to comply with all applicable regulations and restrictions related to the products provided by Qualitides.
Certificates
x- Choosing a selection results in a full page refresh.











